Within our Discovery Research Team we collaborate with several Scientific Research Centers in European area.
Our scientific teams combine a strong know-how gained from TIGEM’s 20+ years of experience in the sector and a continuous innovation in the field.
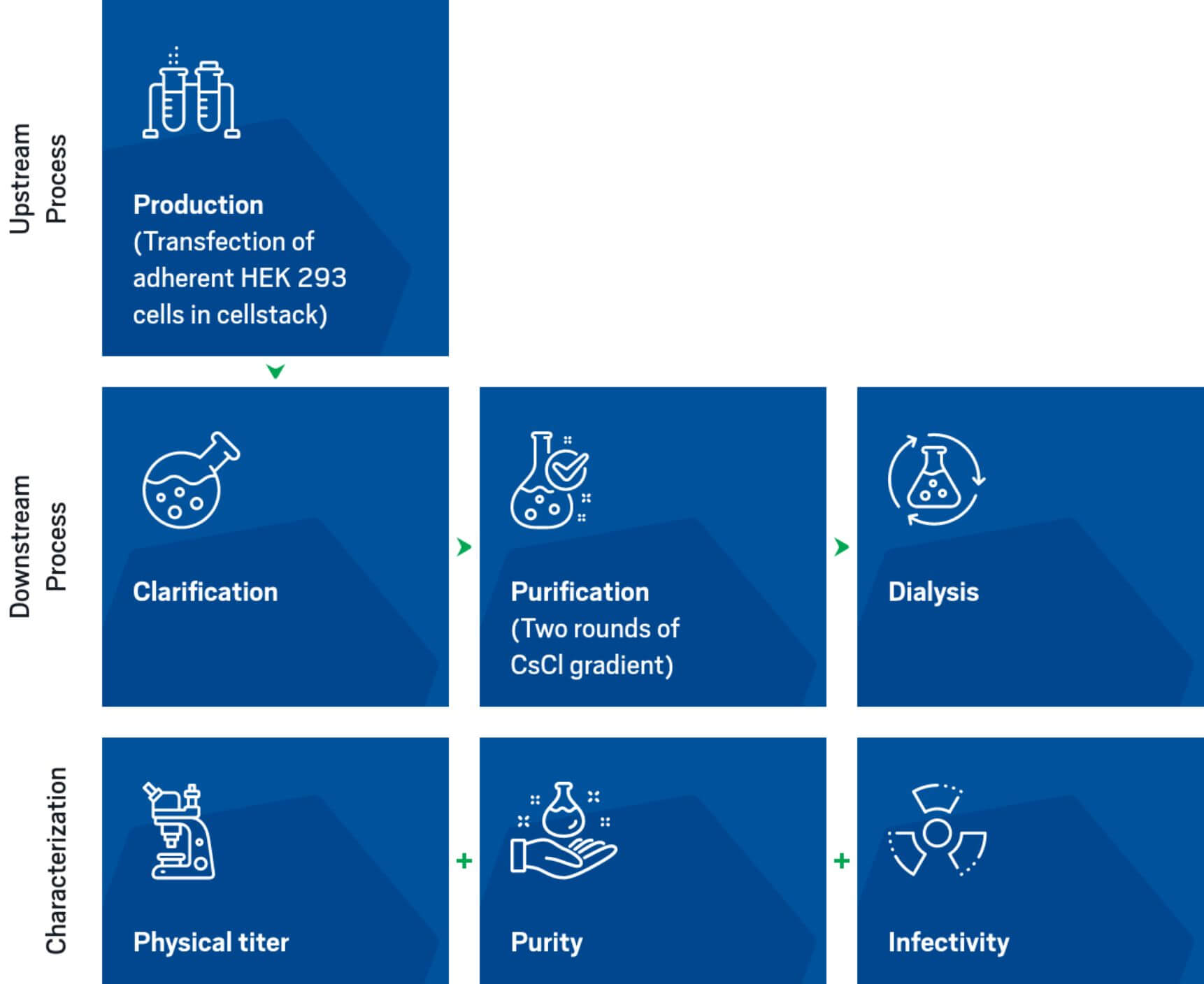
Through our Discovery Research Service we provide fast access to AAV-based product development and manufacturing, starting from the earliest research stages.

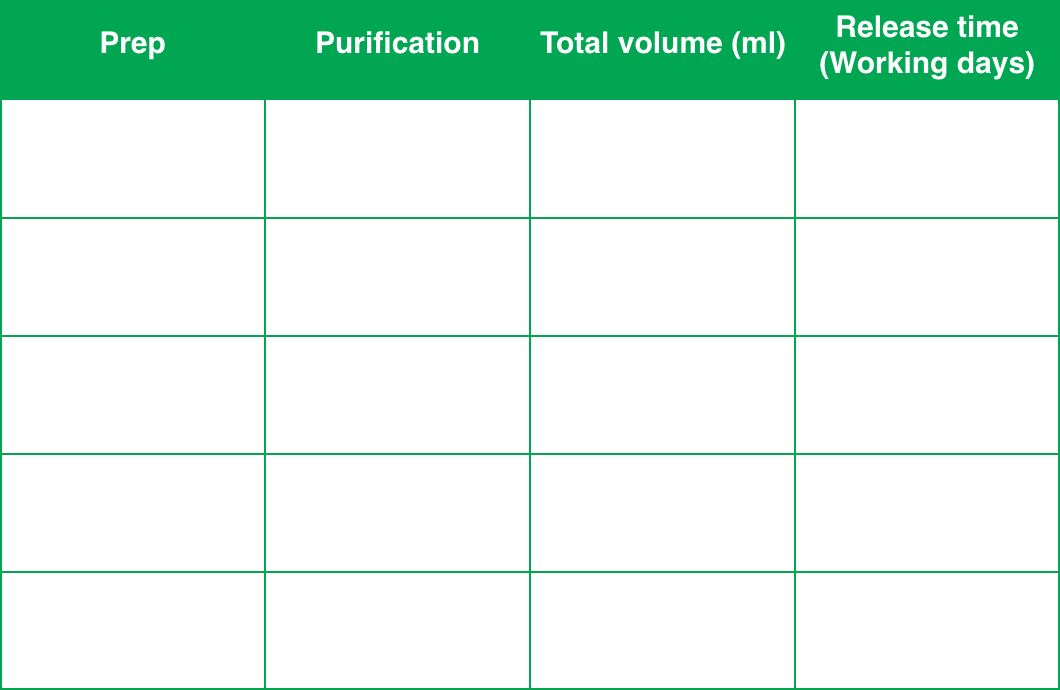
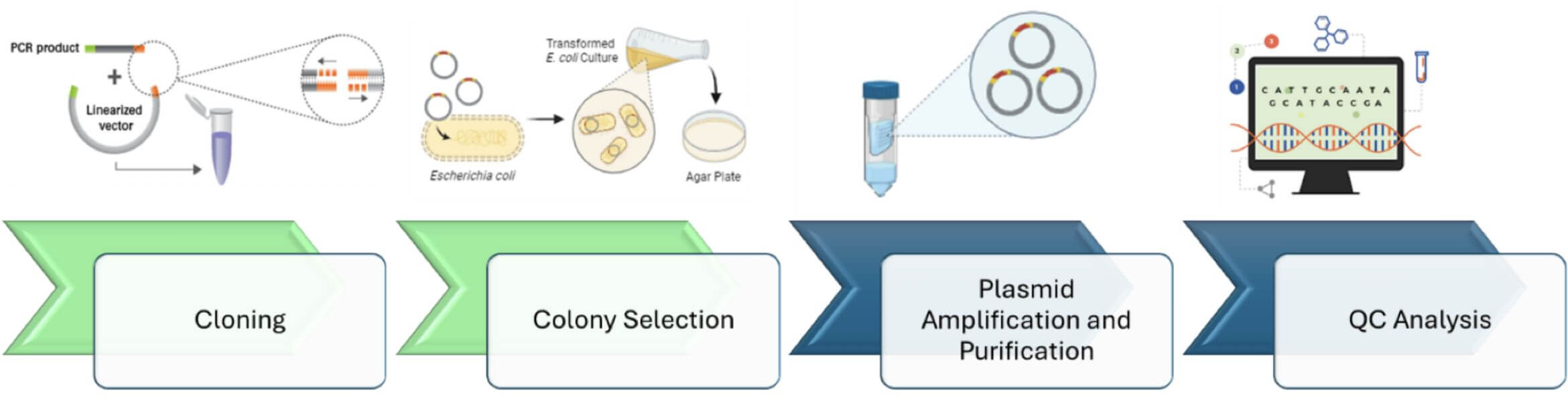
R&D Plasmid cloning
and Production
InnovAAVector s.r.l. provides plasmid cloning and amplification services that are ideal for research grade productions. Regarding bigger scales, for both preclinical and clinical use, InnovAAVector s.r.l. relies on solid collaborations with several plasmid suppliers.

InnovAAVector s.r.l. provides AAVs for all common AAV serotypes. The chart below gives a summary of the tropism of AAV serotypes, indicating the optimal serotype(s) for transduction of a given organ.


Discovery research FAQ
What information do you need from me to produce my vector?
Please download our AAV Production Order form. This form needs to be completed for us to manufacture your custom vector. Please include the following information:
• AAV serotype to be produced.
• Promoter name and source (e.g., human, mouse, viral, etc.).
• Transgene name and source (e.g., human, mouse, etc.).
• Biological activity of transgene.
• PolyA sequence (if applicable).
• The size of the expression cassette from 5’ITR to 3’ITR.
• Other significant map details, such as intron, enhancer, etc.
How do I prepare my AAV cis plasmid for shipping?
Purify your plasmid using an endotoxin-free kit.
Send at least 1200 μg of plasmid packaged with ice packs, with the tube well shielded from breakage.
Verify the integrity of the inverted terminal repeats (ITRs).
Verify the integrity of the expression cassette using restriction enzyme mapping and/or sequencing.
Optional, but recommended: Do a transient transfection of your plasmid into an appropriate cell line to confirm transgene expression and/or function.
Can you send me a cis cloning plasmid that I can use to create the vector plasmid?
Yes, we have cis cloning plasmids into which you can clone your cDNA or your expression cassette.
What are the limitations on the transgene?
• The total amount of sequence that can be packaged into an AAV is approximately 4.8 kb.
• The ITRs take up approx. 300 bp, leaving about 4.5 kb of foreign sequence to be inserted.
• The exact size of transgene depends on the size of your promoter, polyA, and any other sequences you include (such as an intron).
If I use self-complementary AAV (scAAV), what are the limitations?
scAAV (also known as double-stranded AAV (dsAAV)) is produced from cis plasmids containing a deletion in one of the two terminal resolution sites (trs).
Because scAAV is double-stranded, its genome size is approximately half the size of that packaged in single-stranded AAV, corresponding to approximately 2 kb of foreign sequence.
Can you provide vectors with standard reporter genes?
InnovaVector can provide vectors expressing lacZ or EGFP from the ubiquitous CMV promoter.